At 525 C, the equilibrium partial pressures for the substances in the reaction 2 SO₂ (g) + O₂(g) 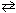 2 SO3(g) were:
2 SO3(g) were:
P SO = 0.0150 atm, P SO = 0.253 atm, and P O = 0.365 atm. The value of K p for this temperature is:
Definitions:
Schizophrenia
A chronic psychological disorder characterized by a disconnection among cognition, affect, and conduct, resulting in distorted perceptions and unsuitable behaviors.
Pessimistic Explanatory Style
A mental approach where individuals habitually attribute negative events to personal, permanent, and pervasive causes.
Setbacks
Events or conditions that hinder progress or cause a decline in performance, often temporarily.
Personal Flaws
Individual weaknesses or shortcomings that are often character-based and can impact one's decisions or actions negatively.
Q5: For the reaction, Ag 3 PO<sub>4</sub> (s)
Q17: Consider the following endothermic reaction at equilibrium:<br>2
Q33: Which of the following would have an
Q45: Consider the following reaction at 1200 K:<br>CH<sub>4</sub>
Q46: What is the molality of a solution
Q72: Exhibit 16-3 Consider the Titration curve below
Q83: Which of the following reactions is product
Q128: Refer to Exhibit 13-1. What is the
Q144: Lead(II) chloride has limited solubility in water
Q145: Refer to Exhibit 13-18. What intermediate (