At 700 C, the equilibrium constant ( K c ) for the reaction
N₂(g) + 3 H₂ (g) 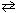 2 NH₃(g) is 2.37 10 - 3 .
2 NH₃(g) is 2.37 10 - 3 .
If 0.683 mol of N₂, 8.80 mol of H₂ and 0.744 mole of NH₃are mixed in a 1.00 L container at 700 C:
Definitions:
Released
The action of making something available to the public, such as a product, movie, or software.
Variable Costing
An accounting method that includes only variable production costs (direct labor, direct materials, and variable manufacturing overhead) in product cost calculations.
Unit Product Cost
The cost calculated by summing all expenses (material, labor, and overhead) associated with producing a single unit of a product.
Fixed Manufacturing Overhead
Indirect manufacturing costs that remain constant regardless of the volume of production, such as salaries of supervisors and rent for factory space.
Q12: The stacking of closest packing layers for
Q19: Iodine-123 is used to study thyroid gland
Q43: One liter of solution is made containing
Q46: What is the conjugate acid of the
Q65: The solubility limit for Sodium Chloride at
Q68: What is the equilibrium constant expression for
Q95: For most substances, the enthalpy of the
Q116: The presence of a nonvolatile solute in
Q123: What is the value of the concentration
Q192: What is the boiling point for a