Consider the reaction:
PCl 3 (g) + Cl₂ (g) 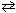 PCl5 (g) K c = 4.2*10- 2
PCl5 (g) K c = 4.2*10- 2
A chemist charged a 1.00 liter reaction vessel with 0.125 moles of PCl5 , 0.250 moles of Cl₂ and 0.375 moles of PCl 3 . If the equilibrium constant, K c , for this reaction is 4.2 10 - 2 , what is the reaction quotient, Q c , and what can be stated about the direction in which this reaction is occurring?
Definitions:
Economic Decline
Economic decline refers to a period when a country's or region's economy diminishes in performance, characterized by reduced GDP, unemployment, and financial instability.
Public Faith
The trust and confidence that the public places in its government and institutions, essential for the stability and effectiveness of a democratic society.
Blue-Collar
Pertaining to workers who engage in manual labor, often in industries such as manufacturing, construction, and maintenance.
Ethnic Voters
Refers to voters grouped by their ethnic background, often considered by political analysts and parties for their potential influence on electoral outcomes.
Q3: What is the definition for molality ?<br>A)
Q14: Consider the following first order reaction for
Q19: Arrange the following aqueous solutions in order
Q31: Calculate the pOH of a solution made
Q36: To calculate the pH of a system
Q59: At 120 ° C, the vapor pressure
Q83: How many grams of sodium acetate, CH<sub>3</sub>COONa,
Q87: The reaction 2 O<sub>3</sub>→3 O<sub>2</sub> has the
Q105: How many grams of NaCl must be
Q140: Refer to Exhibit 13-8. What is the