Consider the Haber process for preparing ammonia from nitrogen and hydrogen as shown below and the reaction s corresponding equilibrium constant at 532 C.
N₂(g) + 3 H₂ (g) 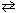 2 NH₃(g) K eq (532 C) = 0.19
2 NH₃(g) K eq (532 C) = 0.19
At a particular moment, the following equilibrium concentrations were observed:
N₂= 0.079 M, H₂ = 0.12 M and NH₃= 0.0051 M. After calculating the reaction quotient , Q , what can be stated about the direction in which this reaction is proceeding?
Definitions:
Put Option
An economic arrangement allowing the holder the option, without being compelled, to sell a designated quantity of an underlying asset at an agreed-upon price within a defined period.
Exercise Price
The price at which the holder of an options contract may buy (in the case of a call) or sell (in the case of a put) the relevant security or commodity.
Expiration Date
In finance, the date at which a derivative contract such as an option or futures agreement ceases to exist.
Tax-Exempt Bonds
Bonds issued by government entities that offer interest payments not subject to federal income tax, and in some cases, state and local taxes.
Q19: Given the following two reactions and their
Q45: A dilute aqueous solution of an organic
Q61: For which of the following processes will
Q72: Which of the following processes is(are) considered
Q78: Given the three statements below, pick the
Q91: At 500 C the equilibrium concentrations are
Q107: What is the definition for molarity ?<br>A)
Q131: Refer to Exhibit 15-6. What is the
Q158: What is the boiling point of an
Q192: Which system at equilibrium shifts right ?<br>I.