Given the following two reactions and their corresponding equilibrium constants, K c , at 298 K:
1 / 2 N₂(g) + 1 / 2 O₂ 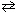 NO (g) K c1 = 6.49*10- 16 2 NO₂ (g)
NO (g) K c1 = 6.49*10- 16 2 NO₂ (g) 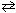 2 NO (g) + O₂(g) K c2 = 4.25*10- 13 What would be the equilibrium constant , K c , for the net reaction given below?
2 NO (g) + O₂(g) K c2 = 4.25*10- 13 What would be the equilibrium constant , K c , for the net reaction given below?
N₂(g) + 2 O₂(g) 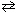 2 NO₂ (g) K c, net = ??
2 NO₂ (g) K c, net = ??
Definitions:
Diaphoretic
Pertaining to sweating, especially profuse sweating, as a medical symptom or bodily response.
Temperature Variation
Fluctuations in temperature over a certain period or across different locations.
Infection
Invasion of the body by pathogenic microorganisms that reproduce and multiply.
Brachial Artery
A major blood vessel of the upper arm that supplies blood to the arm and hand.
Q53: What is the molar solubility of Ag<sub>2</sub>SO<sub>4</sub>
Q59: What is the equilibrium constant expression for
Q93: Exhibit 11-2 The phase diagram below is
Q107: Refer to Exhibit 13-5. The order in
Q131: The solubility of gases in a liquid
Q156: Refer to Exhibit 14-4. What equilibrium expression
Q156: What is the conjugate acid of HSO<sub>4</sub><sup>1
Q179: If we compare the acid/base properties of
Q197: A 0.100 m solution of ZnCl<sub>2</sub> in
Q210: What would be the equilibrium constant expression