Which of the following reactions would shift to the right to favor the product side upon increasing the pressure (through a reduction in the volume of the reaction vessel) ?
I. H₂ (g) + C₂ N₂(g) 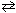 2 HCN (g)
2 HCN (g)
II. CO (g) + Br₂(g) 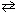 CO Br₂(g)
CO Br₂(g)
III. 6 CO₂ (g) + 6 H₂ O ( 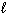 )
) 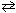 C6 H12 O6 (s) + 6 O₂(g)
C6 H12 O6 (s) + 6 O₂(g)
Definitions:
Patricia A. King
A notable figure in bioethics, often recognized for her contributions to discussing issues of race, gender, and health disparities.
Disease
A condition that impairs the normal functioning of an organism, often characterized by specific signs and symptoms.
Biologically Distinct
Refers to clear differences in the biological characteristics of individuals or groups, often used in the context of species, genetics, or physiological processes.
Q1: Refer to Exhibit 13-8. What is the
Q21: Which of the following reaction(s) at equilibrium
Q31: Calculate the pOH of a solution made
Q73: The reaction 2 NaHSO₄(s) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TBX8714/.jpg" alt="The
Q89: What is the molar solubility of AgIO<sub>3</sub>
Q98: The rate constant for the first order
Q106: Given the following data:<br>2 Cr (s) +
Q114: Which salt(s) listed below form(s) an acidic
Q117: A solution is prepared by mixing 22
Q190: How many milliliters of water must be