Which of the following reaction(s) at equilibrium would shift to the right upon subjecting it to the conditions stated?
I. Increasing the total pressure of the following reaction mixture by introducing an inert gas such as helium:
2 SO₂ (g) + O₂(g) 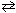 2 SO3 .
2 SO3 .
II. Increasing the pressure of the following reaction mixture through a reduction in volume of the reaction vessel:
H₂ (g) + I2 (g) 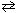 2 HI (g) .
2 HI (g) .
III. Decreasing the pressure of the following reaction mixture through an expansion in volume of the reaction vessel:
Co(H₂ O) 6 2+ (aq) + 4 Cl - (aq) 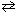 CoCl42 - (aq) + 6 H₂ O (
CoCl42 - (aq) + 6 H₂ O (  )
)
Definitions:
Inflationary Gap
Occurs when equilibrium GDP is greater than full-employment GDP.
Equilibrium GDP
The level of Gross Domestic Product where aggregate supply equals aggregate demand, indicating a stable economy with no tendency for change in the price level or output.
Multiplier
Any change in spending (C, I, or G) will set off a chain reaction leading to a multiplied change in GDP. Equation is 1/(1 - MPC).
Federal Budget Deficit
A situation where the government's expenditures exceed its revenues over a specific fiscal period.
Q9: What intermolecular force(s) of attraction is(are) present
Q83: What is the pH of a 2.6
Q116: Consider the following reaction:<br>2 HBr (g) <img
Q129: Which of the following reactions would shift
Q137: Concentrated red fuming Nitric acid, HNO<sub>3</sub>, is
Q142: Refer to Exhibit 14-5. What would be
Q144: Which of the following is considered a
Q156: Potassium fluoride is used for frosting glass.
Q181: What is the solubility product constant, K
Q189: Consider the following reaction:<br>H₂ (g) + I<sub>2</sub>