For the reaction I2 (g) + Cl₂ (g) 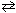 2 ICl (g) at equilibrium, an increase in the volume of the reaction vessel would:
2 ICl (g) at equilibrium, an increase in the volume of the reaction vessel would:
Definitions:
Sample Size
The number of participants or observations included in a study, which impacts the study's ability to detect effects and generalize findings.
Range of Scores
The difference between the highest and lowest values in a set of numerical data, indicating the spread or dispersion of the scores.
Research Method
A systematic plan for conducting research, involving techniques and steps to collect and analyze information to increase our understanding of a topic or issue.
Experimenter Bias
A form of bias introduced by the experimenter whose expectations about the outcome of the experiment can be subtly communicated to the participants.
Q13: A solution was prepared such that the
Q22: What is the mole fraction of lactose
Q63: The reaction PCl<sub>3</sub> (g) + Cl<sub>2</sub> (g)→PCl<sub>5</sub>
Q69: Regarding acid strength, which of the following
Q69: Exhibit 11-2 The phase diagram below is
Q95: What is the criterion for a reaction
Q146: Calculate the boiling point of a solution
Q149: What is the molality of a solution
Q168: Two moles of SO₂ , two moles
Q190: At 1000 K, the value of the