Multiple Choice
The reaction of chlorine and water vapor was allowed to reach equilibrium at 125 C:
2 Cl₂ (g) + 2 H₂ O (g) 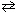 4 HCl (g) + O₂(g) If the volume of the reaction chamber were suddenly doubled, at equilibrium:
4 HCl (g) + O₂(g) If the volume of the reaction chamber were suddenly doubled, at equilibrium:
Definitions:
Related Questions
Q9: A solution contains F<sup> - </sup> and
Q12: Which of the following chemical reactions has
Q20: Refer to Exhibit 13-8. What is the
Q54: A reaction vessel is charged with 1.720
Q57: Given the three molecules listed below, which
Q73: A buffer was prepared by mixing 0.60
Q81: What is the pH after the addition
Q95: Calculate the pH at the equivalence point
Q127: The hydronium ion concentration, [H<sub>3</sub>O<sup>+</sup>], for a
Q136: Which of the following salts is(are) considered