A reaction vessel is charged with 1.720 atm of N₂O4 and 1.220 atm of NO₂ at 25 C. The equilibrium reaction is given in the equation below. N₂O4 (g) 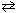 2 NO₂ (g)
2 NO₂ (g)
After equilibrium is reached, the partial pressure of NO₂ is 0.555 atm. What is the value for the equilibrium constant, K c , of this reaction at 25 C?
Definitions:
Legal Disputes
Disagreements or conflicts that are subject to resolution through the legal system, including courts or arbitration.
Peremptory Challenges
The right in jury selection for the attorneys to reject a certain number of potential jurors without stating a reason.
Quasi In Rem
A legal term referring to a court's power over a property or status of an out-of-state defendant's property within its jurisdiction.
Attachment Jurisdiction
The legal authority courts have to seize a defendant's property within its territory to secure jurisdiction over a lawsuit or to satisfy a judgment.
Q1: Consider titrating a triprotic acid with standard
Q21: "A spontaneous change is always accompanied by
Q43: Calculate the pH of a solution that
Q101: Given the abbreviated table for selected standard
Q120: Exhibit 13-13 Graphical analysis can be used
Q138: Under what conditions does changing the pressure
Q155: Which base listed below would be the
Q172: What is the hydronium ion concentration, [H<sub>3</sub>O<sup>+</sup>],
Q181: What is the molality of a solution
Q190: How many milliliters of water must be