When the reversible reaction, N₂+ O₂ 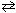 2 NO, has reached a state of equilibrium,
2 NO, has reached a state of equilibrium,
Definitions:
Offeree
The individual or entity to whom an offer is made in a contract scenario.
Offeror
The party in a contract proposal who makes an offer to enter into an agreement, waiting for the acceptance of the offeree.
Assignor
A party who transfers their rights or interests in a contract or property to another party, known as the assignee.
Mirror-Image Rule
A principle in contract law stating that an offer must be accepted exactly without modifications for a valid contract to be formed.
Q12: A 0.250 M aqueous solution of p
Q26: Oxalic acid is diprotic with p K
Q30: Refer to Exhibit 13-11. What is the
Q51: For the high temperature equilibrium system, 2
Q51: What is the vapor pressure of water
Q55: What is the conjugate acid of the
Q76: What is the vapor pressure of a
Q161: A mixture of Na<sub>2</sub>CO<sub>3</sub>, NaCl, and NiCO<sub>3</sub>
Q169: The equilibrium constant K c is 3.00
Q176: The pH of a certain black coffee