At 1400 K, the value of K c for 2 HBr (g) 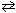 H? (g) + Br?(g) is 1.5*10- 5 . What is the equilibrium concentration of H2 in a 1.00 liter container that initially held 0.10 mol HBr and 0.15 mol of Br?
H? (g) + Br?(g) is 1.5*10- 5 . What is the equilibrium concentration of H2 in a 1.00 liter container that initially held 0.10 mol HBr and 0.15 mol of Br?
Definitions:
Cost Behavior
The way in which a cost reacts to changes in the level of activity.
Fixed Cost
Costs that do not change with the level of production or output, remaining constant even as volume changes.
Relevant Range
The range of activity within which the assumptions about fixed and variable costs are valid. Beyond this range, cost behaviors may change.
Per Unit Basis
A method of cost allocation or measurement that divides a total by the number of units to find the cost per unit.
Q7: A 20.0 mL sample of lactic acid
Q33: The solubility product constant for Ca<sub>3</sub>(PO<sub>4</sub>)<sub>2</sub> is
Q75: Which of the following processes results in
Q84: The concentration of O<sub>2</sub> (g) dissolved in
Q90: The titration of a weak acid, HA
Q103: Which aqueous solution listed below would have
Q126: The best colligative property to use in
Q164: Some N₂H<sub>4</sub> is placed in a 1.0
Q203: The equilibrium constant K c is 10
Q205: Consider the reaction:<br>2 HI (g) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TBX8714/.jpg"