K p at a certain temperature for PCl5 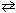 PCl 3 (g) + Cl₂ (g) , is 4.0 10 - 2 . PCl5 is placed into a 1.0 L flask. At equilibrium the partial pressure of PCl 3 = 0.15 atm. What is the partial pressure of PCl5 ?
PCl 3 (g) + Cl₂ (g) , is 4.0 10 - 2 . PCl5 is placed into a 1.0 L flask. At equilibrium the partial pressure of PCl 3 = 0.15 atm. What is the partial pressure of PCl5 ?
Definitions:
Transfer
The act of moving assets, responsibilities, or employees from one place, position, or ownership to another within or between organizations.
Economic Turmoil
A state of significant instability and uncertainty within the economy, often characterized by rapid changes in market conditions, high unemployment, and financial instability.
Legal Issues
Disputes or matters that require resolution through the legal system, involving laws, regulations, and judicial decisions.
Labour Shortages
A situation in the labor market where the demand for workers surpasses the supply, leading to difficulties in finding suitable employees for open positions.
Q44: A 25.0 mL volume of 0.100 M
Q52: Osmosis measures the natural tendency for two
Q71: Which of the following substances would behave
Q95: Refer to Exhibit 13-16. How long would
Q105: How many grams of NaCl must be
Q109: Which substance listed below would be most
Q133: What is the pH of a 0.20
Q138: How many grams of glucose (C<sub>6</sub>H<sub>12</sub>O<sub>6</sub>) must
Q141: The concentration of N<sub>2</sub> (g) dissolved in
Q211: What is the concentration of F<sup> -