The equilibrium constant ( K p ) for the reaction 2 NO₂ (g) 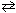 2 NO (g) + O₂(g) is 158 at 1000 K. Calculate the partial pressure of O₂, if the equilibrium partial pressures of NO₂ and NO are 0.400 atm and 0.270 atm, respectively.
2 NO (g) + O₂(g) is 158 at 1000 K. Calculate the partial pressure of O₂, if the equilibrium partial pressures of NO₂ and NO are 0.400 atm and 0.270 atm, respectively.
Definitions:
Binary Tree
A tree data structure where each node has at most two children, often referred to as the left and right child.
Link Instance Variables
Refers to variables within a class instance that refer to other objects, establishing a link between the class and the objects it contains or is associated with.
Data Items
Refers to individual pieces of data or information that are managed or processed by a software program.
References
Variables that hold the memory address of another variable rather than holding the data directly, typically used in languages like C++.
Q10: Which of the following titration curves listed
Q21: What is the acid ionization constant ,
Q22: At 25 ° C, hydrogen iodide decomposes
Q54: What is a half-life ?<br>A) It is
Q72: What species are formed in the hydrolysis
Q87: Calcium fluoride, CaF<sub>2</sub>, has a unit cell
Q90: Consider the reaction given below:<br>H<sub>2</sub> (g) +
Q92: A copper crystal is classified as:<br>A) ionic<br>B)
Q137: At equilibrium, the system N₂O<sub>4</sub> (g) <img
Q208: At 457 K, the value of K