Consider the following reaction:
H₂ (g) + I2 (g) 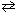 2 HI (g) K eq (448 C) = 50.5 If a reaction vessel is charged witH₂ .00 atm of H₂ and 3.00 atm of I2 , what equation shown below would enable you to solve for x , the change in partial pressures of both H₂ and I2 that accompanies this reaction as it approaches equilibrium?
2 HI (g) K eq (448 C) = 50.5 If a reaction vessel is charged witH₂ .00 atm of H₂ and 3.00 atm of I2 , what equation shown below would enable you to solve for x , the change in partial pressures of both H₂ and I2 that accompanies this reaction as it approaches equilibrium?
Definitions:
Pregnancy
The condition of carrying one or more offspring within the womb, typically lasting around 40 weeks from the last menstrual period to childbirth.
Maternal Mortality Rate
A measure of the number of deaths of women due to pregnancy-related causes per 100,000 live births in a given year or place.
Live Births
The delivery of a baby who shows any sign of life, such as breathing or heartbeat, after separation from the mother's body.
Dilation
The process of widening or expanding an organ or opening in the body, often related to the eyes or blood vessels in response to various conditions.
Q1: When 20.0 grams of a water soluble
Q6: Consider the buffer pair, HF/F<sup> - </sup>.
Q30: A physician studying a mutant, hemoglobin, blood
Q34: What is the volume of 0.0175 M
Q34: K <sub>a</sub> for HF is 3.5×10<sup> -
Q39: What is the equilibrium constant expression for
Q42: Why does temperature affect the rate of
Q44: A 25.0 mL volume of 0.100 M
Q112: How many grams of solute are present
Q125: Which of the following salts is considered