Multiple Choice
Consider the following reaction. Its equilibrium constant is 50.5 at 448 C. H₂ (g) + I2 (g) 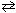 2 HI (g) K p (448 C) = 50.5 If a reaction vessel is charged with 1.00 atm of H₂ and 2.00 atm of I2 , what is the partial pressure of HI at equilibrium ?
2 HI (g) K p (448 C) = 50.5 If a reaction vessel is charged with 1.00 atm of H₂ and 2.00 atm of I2 , what is the partial pressure of HI at equilibrium ?
Understand tools and methods for measuring hydrogen ion concentration.
Compare the effectiveness of different antacids.
Analyze the implications of blood pH changes.
Understand the process of hydrolysis and its importance in breaking down macromolecules.
Definitions:
Related Questions
Q22: Consider the following reaction at equilibrium:<br>2 Cl₂
Q26: At 35 ° C the vapor pressure
Q31: Refer to Exhibit 13-5. The order in
Q53: Benzoic acid is weak with K <sub>a</sub>
Q64: Refer to Exhibit 16-1. What is the
Q74: In a cubic closest packing array of
Q89: A weak acid<br>A) does not react with
Q97: Consider the following unfinished table of weak
Q152: What is the pH of 0.0040 M
Q190: At 1000 K, the value of the