Consider the following system at equilibrium. A reaction vessel is charged witH₂ .46 atm of NO, 2.46 atm of H₂ O and 1.23 atm of H₂ gases. Equilibrium is established as shown in the reaction below. 2 NO (g) + 2 H₂ (g) 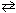 N₂(g) + 2 H₂ O (g) At equilibrium, the partial pressure of NO is 1.53 atm. What is the equilibrium partial pressure of N₂gas in this mixture?
N₂(g) + 2 H₂ O (g) At equilibrium, the partial pressure of NO is 1.53 atm. What is the equilibrium partial pressure of N₂gas in this mixture?
Definitions:
Negative
Referring to something detrimental, unfavorable, or expressing denial, disagreement, or a lack of something.
Informative Message
Communication that aims to provide valuable and useful information to the receiver.
Health Insurance
A type of insurance coverage that pays for medical and surgical expenses incurred by the insured.
Positive Message
A message that conveys optimistic or constructive information or sentiment.
Q15: Calculate the molarity of glucose (C<sub>6</sub>H<sub>12</sub>O<sub>6</sub>) in
Q26: At 35 ° C the vapor pressure
Q64: Refer to Exhibit 16-1. What is the
Q67: Aluminum metal crystallizes in a face centered
Q86: The rate constant of a certain reaction
Q102: Which substance(s) listed below would be expected
Q146: The conjugate acid of HPO<sub>4</sub><sup>2 - </sup>
Q147: The gas-phase reaction shown below follows first
Q151: For the reaction COCl₂ (g) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TBX8714/.jpg"
Q168: What is the molarity of ions in