Which substance(s) listed below would be expected to have a standard molar Gibbs Free-Energy of formation equal to zero, D G f = 0?
I. Na + (aq)
II. O₂(g)
III. H₂ O ( 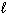 )
)
Definitions:
Marketing Mix
The combination of strategies involving product, price, distribution, and promotion that a company employs to effectively market its goods or services.
Communication
The act of transferring information, ideas, or feelings between individuals or groups using spoken words, written text, or other symbols.
Environmental Forces
External factors that impact an organization's ability to operate, including economic, political, legal, technological, and cultural elements.
Uncontrollable Factors
External elements that influence business operations and success, over which the company has no control, such as economic conditions, social trends, and natural disasters.
Q2: Consider the following Bronsted-Lowry acid-base reaction:<br>C<sub>6</sub>H<sub>5</sub>NH<sub>2</sub> +
Q7: In the azide ion, which has the
Q12: Start with the skeleton half-reaction NO<sub>3</sub><sup> -
Q22: What is the IUPAC name for the
Q31: Which of the following reactions is product
Q36: Sulfide ores are roasted to:<br>A) separate the
Q68: With different ligands a particular transition metal
Q81: Which substance listed below would be expected
Q145: Consider the following Bronsted-Lowery acid-base reaction:<br>HClO ₂
Q178: A Bronsted-Lowry acid is<br>A) a proton acceptor.<br>B)