Exhibit 14-4 Consider the reaction below and its corresponding equilibrium constant at 2000 C to answer the following question(s) :
N₂(g) + O₂(g) 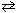 2 NO (g) K c = 4.10 10 - 4 (at 2000 C)
2 NO (g) K c = 4.10 10 - 4 (at 2000 C)
Complete the table shown below for an experiment where 0.35 moles of N₂is mixed with 0.15 moles of O₂in a 1.00 liter reaction vessel and the reaction mixture is allowed to come to equilibrium. Concentration N₂O₂ 2 NO Initial 0.35 M 0.15 M Change At equilibrium
-Refer to Exhibit 14-4. What equilibrium expression shown below would allow you to solve for x and therefore enable you to solve for the equilibrium concentrations of each of the substances present at equilibrium?
Definitions:
Sleep
A naturally recurring state of mind and body characterized by altered consciousness, reduced sensory activity, inhibition of nearly all voluntary muscles, and decreased interactions with surroundings.
Babies
Young infants or very young children, at the earliest stage of their lives.
Activation-Synthesis Hypothesis
is a theory about dreaming that suggests dreams are the result of the brain's attempt to make sense of neural activity that occurs during sleep.
Medulla
Part of the brainstem involved in regulating vital body functions such as heart rate, breathing, and blood pressure.
Q9: Consider splitting water into hydrogen and oxygen
Q23: K <sub>a</sub> for HF is 3.5×10<sup> -
Q28: Consider the following reaction at 730 K:<br>H₂
Q36: What is the molality of a 10.0%
Q59: What is the equilibrium constant expression for
Q64: Rank the following three acids in terms
Q89: The basic repeating three-dimensional pattern of a
Q156: What is the conjugate acid of HSO<sub>4</sub><sup>1
Q161: A dilute solution of HBr is found
Q162: An equilibrium mixture at 500 K has