Consider splitting water into hydrogen and oxygen gas and its corresponding thermodynamic data as shown below:
2 H₂ O ( 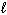 ) 2 H₂ (g) + O₂(g) D S rxn = 326 J/mol K and D H rxn = 572 kJ/mol At what approximate temperature does this reaction cross-over from being considered non-spontaneous to being spontaneous?
) 2 H₂ (g) + O₂(g) D S rxn = 326 J/mol K and D H rxn = 572 kJ/mol At what approximate temperature does this reaction cross-over from being considered non-spontaneous to being spontaneous?
Definitions:
Physical Similarities
The extent to which objects or individuals share common physical characteristics or attributes.
Product Mix
The total range of products or services that a company offers to its customers.
Electronic Retailer
A business that sells goods or services over the internet, allowing customers to shop online from a website or mobile app.
Q16: Calculate the number of moles of sodium
Q23: The half-life of strontium - 90 is
Q26: Which coordination compound can exhibit geometric (
Q38: Given the following Bronsted-Lowery acid-base reaction and
Q39: The decay rate of a beta emitter
Q41: Consider the following reaction and its corresponding
Q42: For 2 N<sub>2</sub>O (g) + O<sub>2</sub> (g)→4
Q44: A sample containing Fe<sub>2</sub>O<sub>3</sub> is dissolved and
Q108: Refer to Exhibit 15-4. What is the
Q153: Calculate the pH of 0.010 M NH<sub>4</sub>Cl.