Exhibit 14-5 Consider the decomposition reaction of Hydrogen sulfide, H₂ S, and the corresponding equilibrium constant as shown below to answer the following question(s) :
2 H₂ S (g) 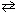 2 H₂ (g) + S2(g) K c = 9.30*10- 8
2 H₂ (g) + S2(g) K c = 9.30*10- 8
Complete the following ICE table for when 0.150 moles of H₂ S is placed in a 1.00 liter container. Molar Conc, M H₂ S H₂ S2Initial Change At equilibrium
-Refer to Exhibit 14-5. What equilibrium expression shown below would allow you to solve for x and therefore enable you to solve for the equilibrium concentrations of each of the substances present at equilibrium?
Definitions:
Actual Output
The real quantity of goods or services produced by a company or a production process, as opposed to planned or theoretical output.
Analysis
The process of examining data or details in order to understand or explain them, typically used to solve problems or make decisions.
Costs Equals Revenues
This refers to a break-even point where the amount of money a company makes from selling goods or services equals the amount it costs to produce them.
Break-even Analysis
An examination to determine the point at which revenue received equals the costs associated with receiving the revenue.
Q3: What is the pH of a solution
Q16: What is the strongest type of intermolecular
Q46: What is the conjugate acid of the
Q76: What is the vapor pressure of a
Q134: The molality of an unknown aqueous solution
Q140: When the reversible reaction, N₂+ O₂ <img
Q141: Which of the following acids is considered
Q151: What is the pH of 2.7 M
Q154: Which of the following reactions is product
Q155: A scientist places 0.0050 mol of H₂