Exhibit 14-6 Consider the reaction at equilibrium below and its corresponding equilibrium constant to answer the following question(s) .
2 NO₂ (g) 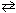 2 NO (g) + O₂(g) K p = 4.48*10- 13
2 NO (g) + O₂(g) K p = 4.48*10- 13
A pressure of 0.45 atm of NO₂ is introduced into a container and allowed to come to equilibrium. Complete the ICE equilibrium table shown below to get started. Partial pressure S2NO₂2 NO O₂Initial 0.45 M Change At equilibrium
-Refer to Exhibit 14-6. What relationship listed below can be used to solve for x in this table and ultimately the equilibrium partial pressures of each substance?
Definitions:
Physical Abuse
The use of physical force that may result in bodily injury, pain, or impairment to an individual.
Eratic Discipline
Inconsistent or unpredictable disciplinary practices.
Schizoid Personality Disorder
A personality disorder characterized by detachment from social relationships and a restricted range of emotional expression.
Narcissistic Personality Disorder
A mental condition characterized by a long-term pattern of exaggerated self-importance, the need for excessive attention and admiration, and a lack of empathy for others.
Q1: When 20.0 grams of a water soluble
Q14: A quantity of PCl<sub>5</sub> gas is heated.
Q30: Refer to Exhibit 13-11. What is the
Q60: Using the entropy values in the table
Q89: Refer to Exhibit 13-3. What is the
Q142: What mass of glycerol (C<sub>3</sub>H<sub>8</sub>O<sub>3</sub>) must be
Q145: For the reaction NH ₄NO₂ (s) <img
Q152: What is the pH of 0.0040 M
Q153: Calculate the pH of 0.010 M NH<sub>4</sub>Cl.
Q170: Which statement below is true about an