Using the entropy values in the table below, what is the change in standard entropy, D S , for the following reaction?
2 CH₃ OH (g) + 3 O₂(g) 2 CO₂ (g) + 4 H₂ O (g) Substance S (J/mol K) CH₃ OH ( 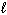 ) 126.8 CH₃ OH (g) 237.6 O₂(g) 205.0 CO₂ (g) 213.6 H₂ O (g) 188.83 H₂ O (
) 126.8 CH₃ OH (g) 237.6 O₂(g) 205.0 CO₂ (g) 213.6 H₂ O (g) 188.83 H₂ O ( 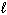 ) 69.91
) 69.91
Definitions:
Laboratory Rats
Rodents that are bred and kept for scientific research.
Ventromedial Hypothalamus
A part of the hypothalamus in the brain involved in regulating hunger, satiety, and the body's energy balance.
Glucose Levels
The concentration of glucose present in the blood, crucial for energy supply to the body's cells.
Neural Circuits
Networks of interconnected neurons that work together to perform specific functions or behaviors in the brain and nervous system.
Q12: Which of the following chemical reactions has
Q13: The binding energy per nucleon is greatest
Q21: In the titration of 25.0 mL of
Q36: Consider the reaction given below:<br>N₂O<sub>4</sub> (g) <img
Q49: Given the abbreviated table for selected standard
Q51: The transformation of <sup>14</sup>C→<sup>14</sup>N is an example
Q58: What is the pH of an aqueous
Q83: Which of the following reactions is product
Q84: The following equilibrium is established in water
Q116: Consider the following reaction:<br>2 HBr (g) <img