The equilibrium constant K p for the reaction
NH ₄ CO₂ NH₂ (s) 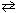 2 NH₃(g) + CO₂ (g) is 2.5*10- 4
2 NH₃(g) + CO₂ (g) is 2.5*10- 4
At 310 K. Calculate the pressures (in atm) of the two gases in equilibrium with excess solid NH ₄ CO₂ NH₂ at 310 K.
Definitions:
Ethical Considerations
The process of evaluating decisions and actions based on moral values and principles.
Profit Maximization
A strategic goal of businesses to achieve the highest possible profit through revenue generation and cost management.
Business Decision
A choice or judgement made as part of managing a company, affecting its direction, operations, and strategy.
Duty-Based
An ethical framework that focuses on the obligations one has towards others, emphasizing the performance of duties as morally right actions.
Q6: The K <sub>sp</sub> of Mg<sub>3</sub>(PO<sub>4</sub>)<sub>2</sub> is 1.0×10<sup>
Q71: Calculate the osmotic pressure of a solution
Q92: The definition of a Lewis base is:<br>A)
Q122: What is the equilibrium partial pressure of
Q124: What is the pH of a solution
Q133: The following are factors that influence the
Q143: At a particular temperature, the solubility of
Q175: A researcher places 0.10 mol of CO
Q186: Consider mixing 5.1×10<sup> - 6</sup> moles of
Q196: How many grams of urea [CO(NH<sub>2</sub>)<sub>2</sub>] would