Consider solving for the molar solubility of a 3:1 electrolyte such as Ag3PO4 . Ag3PO4 (s) 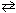 3 Ag 1+ (aq) + PO4 3 - (aq)
3 Ag 1+ (aq) + PO4 3 - (aq)
After constructing an ICE table and writing a solubility constant expression for this process, what solubility product expression listed below would be suitable for solving for x which could then be related to the equilibrium concentrations of Ag 1+ and PO4 3 - ion concentrations and through stoichiometry can be related to the solubility of Ag 3 PO4 in water?
Definitions:
Csikszentmihalyi
A psychologist known for his work on the concept of flow, a state of absorption where one's skills are fully involved in overcoming a challenge that is just about manageable.
Left-brained
Referring to a person who is more analytical, logical, and detail-oriented, traditionally thought to be associated with the left hemisphere of the brain.
Right-brained
Pertaining to the cognitive style thought to be characteristic of the brain's right hemisphere, often associated with creativity, intuition, and holistic thinking.
Stadium Seating
A seating arrangement designed to give spectators in stadiums an unobstructed view of the event by arranging seats in ascending rows.
Q11: Refer to Exhibit 16-2. What compound(s) is(are)
Q23: Consider the following unbalanced reaction :<br>NO (g)
Q54: Which aqueous solution has the lowest freezing
Q55: The mathematical form of the first law
Q81: Which substance listed below would be expected
Q86: The density of a solution that is
Q116: A 0.20 M solution of the weak
Q123: What is the value of the concentration
Q144: Which of the following is considered a
Q197: A 0.100 m solution of ZnCl<sub>2</sub> in