Given the following Bronsted-Lowery acid-base reaction and its corresponding equilibrium constant, which substance is the strongest acid ?
CH ₃CH ₂ NH₃+ + ClO21 - 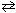 CH ₃CH ₂ NH₂ + HClO ₂ K = 2.1 10 - 9
CH ₃CH ₂ NH₂ + HClO ₂ K = 2.1 10 - 9
Definitions:
Asset Improvements
Enhancements or upgrades made to a company's assets to increase their efficiency, productivity, or value.
Ordinary Maintenance
Ordinary maintenance involves the routine upkeep and repairs necessary to maintain assets in their current condition, without significantly enhancing their value or extending their life.
Asset Improvements
Expenditures made to increase the service life, operating efficiency, or productive capacity of an asset.
Ordinary Maintenance
Routine upkeep and repairs to keep equipment and facilities in working order, as opposed to major renovations or upgrades.
Q1: At 100 C, the equilibrium constant for
Q5: Refer to Exhibit 13-3. What is the
Q63: Refer to Exhibit 18-2. Which electrode is
Q77: Given D G rxn = - 120.4
Q86: Which of the following is true?<br>A) D
Q96: Which of the following colligative properties is
Q113: A solution that contains 2.00 g of
Q134: The reaction H<sub>3</sub>C - I + OH<sup>
Q168: Refer to Exhibit 15-6. What is the
Q171: Refer to Exhibit 14-7. What is the