Exhibit 13-3 Consider the following reaction and the corresponding time-concentration table to answer the following question(s) . C2H4 (g) + O3 (g) →C2H4O (g) + O2 (g) The concentration of ozone, O3, was monitored for this reaction as a function of time and is given in the table that follows. 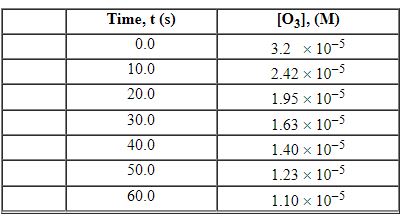
-Refer to Exhibit 13-3. What is the average rate for the time interval from 40.0 seconds tO60 seconds?
Definitions:
Transitional Stage
A phase in development or progress where significant changes occur, leading from one state or condition to another.
Population Regulation
The mechanisms and processes that control the size and growth of a population.
Environmental Problems
Issues affecting the natural world, often caused by human activities, including pollution, climate change, deforestation, and loss of biodiversity.
Social Problems
Issues that adversely affect an individual's or group's well-being within a society, often requiring collective action for resolution.
Q6: What is the freezing point of a
Q17: Consider the following endothermic reaction at equilibrium:<br>2
Q55: What is the electron configuration of NO<sup>
Q64: When the reversible reaction, N₂+ O₂ <img
Q75: Which aqueous solution listed below would be
Q78: A weak monoprotic acid is dissolved in
Q108: An endothermic reaction CaCO<sub>3</sub>(s) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TBX8714/.jpg" alt="An
Q112: How many grams of solute are present
Q128: Refer to Exhibit 13-1. What is the
Q165: The equilibrium constant K p for the