Exhibit 15-4 Consider dissolving NaF in water as shown in the equation below to answer the following question(s) :
NaF (s) + H₂ O ( 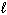 )
) 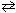 HF (aq) + NaOH (aq)
HF (aq) + NaOH (aq)
-Refer to Exhibit 15-4. What is the base ionization constant , K b , for this reaction?
K a (HF) = 6.8 10 - 4
Definitions:
Millionaires
Individuals whose net worth or wealth equals or exceeds one million units of currency.
College Degrees
Formal certifications provided by colleges or universities indicating the completion of a specific program of study.
Inheritance
The process by which property, titles, debts, rights, or obligations are transferred from one individual to another upon the death of the first individual.
Working Conditions
The environment and terms under which employees perform their jobs, including aspects such as hours, safety, and physical demands.
Q20: Consider a mixture of three gases in
Q21: What is the acid ionization constant ,
Q59: The reaction of ammonia and hydrogen chloride
Q76: What is the effect upon addition of
Q100: A 0.050 M solution of ammonium chloride
Q103: Given:<br>Cu (s) + 2 Ag<sup>+</sup>→2 Ag (s)
Q125: Which of the following salts is considered
Q130: A 0.010 M solution of the strong
Q158: An amphoteric substance:<br>A) has a very low
Q194: At 400 C the value of K