Consider a mixture of three gases in the following reaction and the reaction s corresponding Standard Molar Gibbs Free Energy:
2 HI (g) 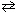 H₂ (g) + I2 (g) D G = 16.8 KJ/mol If the partial pressures of the three gases are P HI = 2.50 atm, PH 2= 3.5 atm and P I2 = 4.5 atm at 400 K, what is D G under these non-standard conditions ?
H₂ (g) + I2 (g) D G = 16.8 KJ/mol If the partial pressures of the three gases are P HI = 2.50 atm, PH 2= 3.5 atm and P I2 = 4.5 atm at 400 K, what is D G under these non-standard conditions ?
Definitions:
Ultrasonic Irradiation
The application of high-frequency sound waves to materials or substances, often used for cleaning, mixing, or accelerating chemical processes.
Mannose-6-phosphorylated
A term related to biochemistry, referring to a mannose sugar molecule that has been phosphorylated at the 6th position.
Parallel Bioreactors
Equipment used concurrently in the cultivation of microorganisms, cells, or biochemical reactions under controlled conditions.
New Information
Knowledge or data that has been recently discovered or revealed, which was previously unknown.
Q4: From the list below, pick the choice
Q21: What is the acid ionization constant ,
Q24: What is the concentration of Pb 2+
Q49: A voltaic cell is made by combining
Q54: Start with the skeleton half-reaction NO<sub>2</sub><sup> -
Q55: When Plutonium - 239 changes to Uranium
Q65: Refer to Exhibit 15-4. What is the
Q68: The pH of a vinegar solution is
Q99: Which of the following systems show(s) a
Q198: Enough NO gas was placed into a