A 0.40 gram sample of NaOH (molar mass = 40.0 g/mol) was completely neutralized witH₆2.5 mL of H₂ SO₄solution. Assume the chemical equation is:
H₂ SO₄(aq) + 2 NaOH (aq) Na2 SO₄(aq) + 2 H₂ O ( 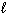 ) The molarity of the H₂ SO₄solution was:
) The molarity of the H₂ SO₄solution was:
Definitions:
Debt/Equity Ratio
A calculation that shows how much a company relies on borrowed funds, found by dividing the sum of its liabilities by the equity owned by shareholders.
Long-term Debt Ratio
A financial ratio that shows the proportion of a company’s long-term debt compared to its total assets.
Total Debt
Refers to the sum of all financial obligations (short-term and long-term liabilities) owed by an individual or entity.
Net Cash
The amount of cash that is available after all debts, expenses, and operational costs have been deducted.
Q7: At 37 ° C the autoionization constant
Q14: A quantity of PCl<sub>5</sub> gas is heated.
Q16: Acid rain is a result of water
Q40: The largest scale commercial use of hydrogen
Q50: Refer to Exhibit 14-6. Upon constructing an
Q56: Which of these metals is not a
Q59: What is the equilibrium constant expression for
Q69: Refer to Exhibit 13-11. What is the
Q126: In the acid-base equilibrium PO<sub>4</sub> 3 -
Q171: Refer to Exhibit 14-7. What is the