Consider the following equilibrium reaction:
NH₃+ H₂ O 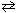 NH ₄+ + OH - K b (NH ₃) = 1.8 10 - 5 Qualitatively, what is the effect of adding NaOH to an aqueous solution of NH₃as shown in the equilibrium reaction above?
NH ₄+ + OH - K b (NH ₃) = 1.8 10 - 5 Qualitatively, what is the effect of adding NaOH to an aqueous solution of NH₃as shown in the equilibrium reaction above?
(Use Le Chatelier s principle.)
Definitions:
Unconditional Promise
A firm commitment or pledge not subject to any conditions or contingencies.
Transferee
An individual or entity that receives property, rights, or interests from another through a legal transfer process.
Endorsement
The act of signing the back of a negotiable instrument, which allows for the transfer of rights from one party to another or specifies particular conditions of transfer.
Conditional Endorsement
An endorsement on a negotiable instrument that imposes conditions on the use or payment of the instrument.
Q18: The equilibrium constant, K p , for
Q18: What is the missing particle in the
Q31: Pick the choice below that is an
Q51: The transformation of <sup>14</sup>C→<sup>14</sup>N is an example
Q74: A 0.40 gram sample of NaOH (molar
Q86: Pyridine (p K <sub>b</sub> = 8.74) reacts
Q102: After balancing the following reaction under acidic
Q141: For the reaction 2 C₂ H₂ (g)
Q147: The gas-phase reaction shown below follows first
Q212: The equilibrium constant ( K p )