Consider the ionization of hydrofluoric acid as follows:
HF + H₂ O 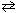 H₃O + + F 1 - What is the effect of adding the common ion, F - (source:
H₃O + + F 1 - What is the effect of adding the common ion, F - (source:
NaF) , to this equilibrium system?
Definitions:
James-Lange Theory
A theory suggesting that emotions occur as a result of physiological reactions to events.
Schachter-Singer Theory
A theory of emotion that states emotions are determined by physiological arousal and the cognitive interpretation of that arousal.
Binge Eating
The consumption of large quantities of food in a short period of time, often associated with a feeling of loss of control over eating.
Anorexia Nervosa
An eating disorder characterized by an abnormally low body weight, an intense fear of gaining weight, and a distorted perception of body weight or shape.
Q16: A nitric acid (HNO<sub>3</sub>) solution is 16.2
Q17: With different ligands a particular transition metal
Q29: Which of the following general combinations of
Q41: What is the solubility of PbSO<sub>4</sub> (
Q60: What is the pH of an aqueous
Q66: What is the pH of an aqueous
Q76: What is the pH of aqueous 0.10
Q80: Consider the buffer pair, NH<sub>4</sub>Cl/NH<sub>3</sub>. What can
Q132: Refer to Exhibit 13-14. If 0.0250 moles
Q140: When the reversible reaction, N₂+ O₂ <img