Consider titrating a triprotic acid with standard NaOH as shown in the titration curve below. 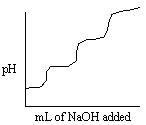 What is the relationship between the first equivalence point and the third equivalence point in this titration curve?
What is the relationship between the first equivalence point and the third equivalence point in this titration curve?
Definitions:
Nutritional Status
An assessment of the adequacy of nutrition and the body’s ability to absorb and utilize nutrients.
Osteomalacia
A softening of the bones due to vitamin D deficiency or impaired metabolism, leading to increased susceptibility to fractures.
Nutritional Status
An assessment of the body's health in terms of nutrient levels, diet, and physical health indicators, used to identify potential deficiencies or imbalances.
Widow
An individual whose spouse has died and who has not remarried.
Q20: What is the IUPAC name for the
Q36: To calculate the pH of a system
Q41: Consider the following reaction and its corresponding
Q43: A reaction will proceed spontaneously at any
Q87: What is the concentration of ammonium ion,
Q109: What species are formed in the hydrolysis
Q118: Arrhenius proposed the equation shown below that
Q153: Calculate the pH of 0.010 M NH<sub>4</sub>Cl.
Q162: An equilibrium mixture at 500 K has
Q166: Identify the Lewis base in the following