Which of the following reactions would be expected to have a positive entropy change, D S 0?
I. 2 SO₂ (g) + O₂(g) 2 SO3(g)
II. Ba(OH) 2 (s) BaO (s) + H₂ O (g)
III. CO (g) + 2 H₂ (g) CH₃ OH ( 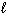 )
)
Definitions:
Fixed Cost
Costs that do not fluctuate in relation to the level of production or sales, such as rent or salaries.
Economic Order Quantity
The optimal quantity of inventory to order that minimizes total inventory costs, including holding, ordering, and shortage costs.
Carrying Cost
The total expense of holding a particular asset over time, including storage, insurance, and taxes, but excluding buying cost.
Order Cost
Expenses incurred in placing and receiving orders from suppliers, including costs related to processing, shipping, and handling.
Q10: With respect to any voltaic cell, at
Q25: The O - O bond order in
Q26: Oxalic acid is diprotic with p K
Q55: In which of the complexes are optical
Q68: Given K p = 0.100 at 25
Q72: Which metal listed below has the highest
Q94: A solution is made by dissolving 0.100
Q112: What is the value of the equilibrium
Q123: What is the value of the concentration
Q167: What is the H<sub>3</sub>O<sup>+</sup> concentration in a