The following reduction of Fe2 O3 to Fe occurs in blast furnaces during the steel making process:
3 CO (g) + Fe2 O3(s) 2 Fe (s) + 3 CO₂ (g) Calculate 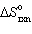 per mole of Fe2 O3 reacted, if the values of S for CO (g) , Fe2 O3(s) , Fe (s) , and CO₂ (g) are 198, 87, 27, and 214 J/mol K, respectively (answer in units of J/K) .
per mole of Fe2 O3 reacted, if the values of S for CO (g) , Fe2 O3(s) , Fe (s) , and CO₂ (g) are 198, 87, 27, and 214 J/mol K, respectively (answer in units of J/K) .
Definitions:
Evaluation
The process of assessing or judging the value, quality, or importance of something based on certain criteria.
Skepticism
An attitude of doubt or a disposition to incredulity either in general or toward a particular object.
Empirical Evidence
Information acquired by observation or experimentation that is used as a basis for knowledge in science.
Observation
The act of carefully watching and noting phenomena as they occur in order to gather data.
Q9: Consider splitting water into hydrogen and oxygen
Q13: Which substance(s) listed below has(have) a standard
Q67: One mole of SO₂ , two moles
Q83: For the reaction, PCl<sub>5</sub> (g) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TBX8714/.jpg"
Q93: The products of an electrolysis may depend
Q96: The best measure of the spontaneity of
Q100: Refer to Exhibit 17-2. Which statement below
Q105: When comparing Bronsted-Lowery acid strengths , which
Q111: Compare compounds I-III with compound A. Which
Q193: The reaction of chlorine and water vapor