Consider the reaction given below:
N₂O4 (g) 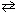 2 NO₂ (g) at 25 C If D G = - 5.39 kJ and K p = 8.81 for this reaction, calculate the value of D G reaction when the partial pressures of NO₂ and N₂O4 are 1.50 atm and 2.40 atm, respectively. R = 8.314 J/mol K
2 NO₂ (g) at 25 C If D G = - 5.39 kJ and K p = 8.81 for this reaction, calculate the value of D G reaction when the partial pressures of NO₂ and N₂O4 are 1.50 atm and 2.40 atm, respectively. R = 8.314 J/mol K
Definitions:
Convenience Sampling
A non-probability sampling method where subjects are selected because of their convenient accessibility and proximity to the researcher.
HR Department
The department within an organization that is focused on activities relating to employees, including hiring, training, compensation, benefits, and workplace culture.
Email Distribution
A strategy used to send a single email or series of emails to a large group of recipients, aiming at marketing, information dissemination, or engagement.
Voluntary Response Sampling
A sample which consists of individuals who choose themselves to participate, leading to potential bias.
Q13: The molecular formula for butene is:<br>A) C<sub>4</sub>H<sub>4</sub><br>B)
Q16: Which of these transition elements has the
Q24: The equilibrium constant for the reaction N₂O<sub>4</sub>
Q34: K <sub>a</sub> for HF is 3.5×10<sup> -
Q59: In the oxidation-reduction equation (balanced):<br>H<sub>2</sub>O + 3
Q79: A buffer solution maintains a constant pH
Q83: Curium - 248, <sup>248</sup>Cm, was bombarded, yielding
Q86: Pyridine (p K <sub>b</sub> = 8.74) reacts
Q89: The reaction below can be best described
Q181: What is the solubility product constant, K