Given the following Bronsted-Lowery acid-base reaction and its corresponding equilibrium constant, which substance is the strongest acid ?
CH ₃CH ₂ NH₃+ + ClO21 - 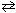 CH ₃CH ₂ NH₂ + HClO ₂ K = 2.1 10 - 9
CH ₃CH ₂ NH₂ + HClO ₂ K = 2.1 10 - 9
Definitions:
Numbers and Statistics
The use of mathematical figures and models to collect, analyze, and interpret quantitative data.
Content Analyses
A research method used to interpret text data through the systematic classification process of coding and identifying themes or patterns.
Critical Analyses
The process of examining an issue or object in detail to evaluate its merits, flaws, and value, often leading to a deeper understanding or interpretation.
Rhetorical
Pertaining to the art of effective or persuasive speaking or writing, often with the use of figures of speech and other compositional techniques.
Q1: Consider titrating a triprotic acid with standard
Q2: Consider the following Bronsted-Lowry acid-base reaction:<br>C<sub>6</sub>H<sub>5</sub>NH<sub>2</sub> +
Q25: The highest oxidation state expected for Ti
Q49: Given the abbreviated table for selected standard
Q56: The oxidation state of chlorine in ClO<sub>4</sub><sup>
Q67: The pH of a 1.00 liter solution
Q141: Which of the following acids is considered
Q163: In the unusual acid-base reaction HClO₄ +
Q187: Which of the items listed below would
Q192: Which system at equilibrium shifts right ?<br>I.