What is the equilibrium constant, K eq , for the following reaction at 25 C, given that D G rxn = - 16.8 kJ/mol?
H₂ (g) + I2 (g) 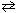 2 HI (g)
2 HI (g)
Definitions:
Poison Pill
Shareholder rights provisions that allow existing shareholders in a company to purchase additional shares of stock at a lower than market value if a potential acquirer purchases a controlling stake in the company.
Corporate Restructuring
The process of significantly changing a company's business model, organizational structure, or financial structure, often in response to financial pressures.
Horizon Value
The estimated value of a company or investment at the end of a specific period, taking into account future cash flows or income it will generate.
Weighted Average
A calculation that takes into account the varying degrees of importance of the numbers in a data set.
Q9: Which metal ion would have the largest
Q10: Which of the following titration curves listed
Q20: An acid HB<sup>+</sup> has a K <sub>a</sub>
Q36: A current is passed through a molten
Q52: The following compounds have very limited solubility
Q64: The redox reaction (unbalanced), Cl<sub>2</sub>→ClO<sub>3</sub><sup> - </sup>
Q78: Consider the following hexamer. What monomer unit
Q83: What is the oxidation number of the
Q99: Refer to Exhibit 22-6. What three functional
Q166: The molecular formula for hexane is:<br>A) C<sub>6</sub>H<sub>6</sub><br>B)