A chemist was performing a spectroscopy experiment on a fluorescent dye to examine the influence of concentration on absorbance. To accomplish this, the chemist created a stock solution of a certain concentration of dye using 10 mL of water (H2O) . Then, a serial dilution was performed by taking 1 mL of the stock solution and diluting it with 9 mL of water for a total solution volume of 10 mL. 1 mL was taken from Solution 2 and diluted with 9 mL water to create Solution 3. The serial dilution was performed through Solution 5, as shown in the diagram below. If the dye concentration in Solution 5 was 0.5 g/mL, what was the concentration of the dye in Solution 2?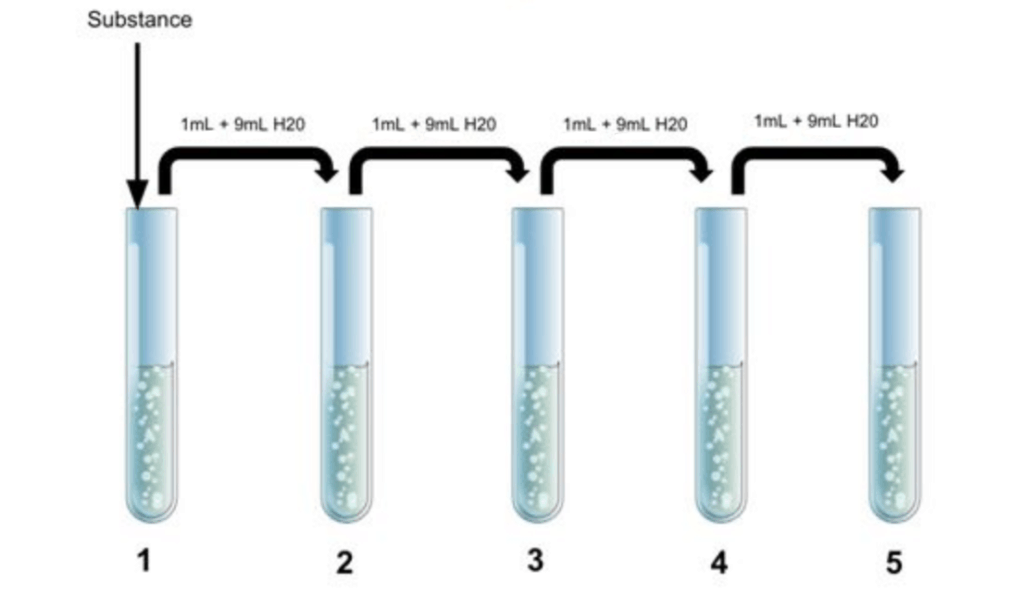
Definitions:
Customers
Individuals or entities that purchase goods or services from a business, playing a central role in its success.
Value
The importance, worth, or usefulness of something to a consumer, often influencing their purchasing decisions.
Ethical Firms
Companies that operate in ways that are morally right, adhering to principles of fairness, honesty, and respect for individuals and the environment.
U.S. Large-Cap Index
An index that tracks the performance of stocks with large market capitalizations in the United States.
Q2: What is the next number in the
Q24: Which of the following Kelvin temperatures represents
Q45: If the first two statements are true,
Q57: The words SOOTHE and AGGRAVATE have _?_
Q149: Which of the following describes the function
Q192: Which of the following statements about protons
Q253: Choose the correct complementary bases for the
Q277: A friend call and tells you, "I
Q338: Which of the following is the result
Q380: Which of the following statements is true?<br>A)DNA