The following reaction represents the self-ionization of ammonia (NH3).
NH3(aq)+ NH3(aq) 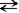 NH2-(aq)+ NH4+(aq)
NH2-(aq)+ NH4+(aq)
amide ammonium
Fill in the blanks with the appropriate terms from those listed below.
ammonia
ammonium
amide
-In the reverse reaction, __________________________ion functions as an acid.
Definitions:
Hawaiian Islands
An archipelago located in the Pacific Ocean, formed by volcanic activity from a hot spot beneath the Earth’s crust.
Aleutian Islands
a chain of islands located in the Northern Pacific Ocean, forming part of the Alaska state boundary.
Seafloor Depth
The vertical distance from the ocean surface to the bottom of the ocean floor, varying greatly across different oceanographic features.
Trench
A long, narrow ditch often used for defense, utilities, or as a significant topographical feature on the ocean floor.
Q2: The following compound cannot be dehydrated to
Q5: If you dissolve 0.10 mol of a
Q7: Using most common charges, the ionic compound
Q11: One would expect a compound with the
Q29: If the 'Nutrition Facts' on a food
Q32: Photosynthesis and respiration are linked to each
Q34: With like molecules, structure _ could participate
Q37: How many millimeters are equivalent to 40.5
Q39: What are the products of the hydrolysis
Q42: _would not increase the H<sub>3</sub>O<sup>+</sup> concentration in