If we know the amount of light that is absorbed in a solution, we can calculate the concentration of the substance dissolved in water using a spectrophotometer. For a certain substance the concentration (in moles/liter) is given by the formula  , where
, where 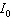 is the intensity of the incident light and
is the intensity of the incident light and 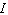 is the intensity of light that emerges. What is the concentration of the substance if the intensity
is the intensity of light that emerges. What is the concentration of the substance if the intensity 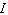 is 45% of
is 45% of 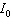 ?
?
Definitions:
Maslow's Hierarchy
A theory in psychology proposed by Abraham Maslow that outlines a five-tier model of human needs, from basic physiological needs to self-actualization.
Self-actualization
The achievement of realizing one's capabilities and talents, seen as an innate drive or necessity in all individuals.
Maturation
The process of development or reaching a state of full growth or potential in biological, psychological, or emotional contexts.
Q6: Which of the following flooring types has
Q7: All photovoltaic cells made of silicon produce
Q7: Industrial systems often use _ to concentrate
Q8: What is the least common type of
Q17: Which of the following statements regarding treated
Q17: For the graph below, find the solution(s)
Q28: The pH of tomatoes is normally in
Q49: Biologists observed that as the temperature t
Q53: A college conducted a survey of students
Q61: If the price of gas is $2.35