If we know the amount of light that is absorbed in a solution, we can calculate the concentration of the substance dissolved in water using a spectrophotometer. For a certain substance the concentration (in moles/liter) is given by the formula  , where
, where 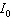 is the intensity of the incident light and
is the intensity of the incident light and 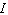 is the intensity of light that emerges. What is the concentration of the substance if the intensity
is the intensity of light that emerges. What is the concentration of the substance if the intensity 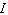 is 45% of
is 45% of 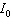 ?
?
Definitions:
MS Excel 2008
A version of Microsoft Excel, a spreadsheet software, released in 2008 with features for calculation, graphing tools, pivot tables, and a macro programming language.
Template
A pre-designed layout or structure used as a starting point for creating documents, presentations, or projects, to ensure consistency and efficiency.
Standard Design Features
Common or typical characteristics, specifications, or elements incorporated into a design to meet minimum requirements or norms.
Example Document
A sample document used as a reference or template, showcasing what should be included or how something is formatted.
Q2: One advantage of the correlated-groups t test
Q3: Tidal barrages _ . <br>A) do
Q16: The initial population of f(t) is equal
Q21: The graph below shows a system of
Q31: The value of <img src="https://d2lvgg3v3hfg70.cloudfront.net/TBX8728/.jpg" alt="The value
Q40: Use the Laws of Exponents to expand
Q44: The cost of college tuition for 12
Q54: Which one of the following functions translates
Q54: Which one of the following is the
Q94: Sally wishes to invest $4500 in a