A Lewis dot structure for benzene, C6H6 would give two equivalent resonance structures for this cyclic molecule with alternating single and double bonds 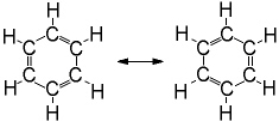 A combined LCAO and MO picture for the bonding of the carbon atoms in benzene would be
A combined LCAO and MO picture for the bonding of the carbon atoms in benzene would be
Definitions:
Net Working Capital
The variance between an organization's immediate assets and its short-term obligations, reflecting its short-term fiscal well-being.
Straight-Line Depreciation
A method of evenly distributing the cost of a tangible asset over its useful life, resulting in a fixed annual depreciation expense.
Required Rate Of Return
The minimum annual percentage earned by an investment that will induce individuals or companies to put money into a particular project or investment.
Tax Rate
The percentage rate at which the government taxes an individual’s or corporation’s income.
Q3: A researcher constructed a reaction vessel and
Q10: Arrange the following in order of increasing
Q18: If the bond in carbon monoxide is
Q19: Tricksters are sometimes referred to as which
Q20: Exhibit 17-2<br>The following question(s) pertain to an
Q22: Exhibit 15-2 The following question(s) pertain to
Q23: You are trying to use percent mass
Q24: Exhibit 14-2<br>The following question(s) pertain to
Q29: Exhibit 16-1 The following question(s) pertain to
Q33: Given that the electronic configuration of Cu