A Lewis dot structure for benzene, C6H6 would give two equivalent resonance structures for this cyclic molecule with alternating single and double bonds 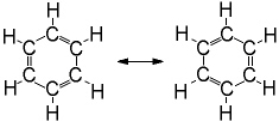 A combined LCAO and MO picture for the bonding of the carbon atoms in benzene would be
A combined LCAO and MO picture for the bonding of the carbon atoms in benzene would be
Definitions:
Downward-Sloping
A term used to describe a line or curve on a graph that moves from the upper left to the lower right, often associated with the demand curve in economics.
Complements
Goods and services that are used together, where the consumption or use of one increases the demand for the other.
Normal Goods
Goods for which demand increases as consumer income rises, holding prices constant.
Steak
A cut of meat, typically beef, that is sliced perpendicular to the muscle fibers.
Q3: Which of the following is a tertiary
Q11: Exhibit 19-2<br>The following question(s) pertain to a
Q14: Which of the following is true for
Q19: Fifty grams of solid I<sub>2</sub> is placed
Q23: The decomposition of NO and NO<sub>2</sub> to
Q28: For the one dimensional particle in a
Q29: The destruction of ozone (O<sub>3</sub>) typically takes
Q32: Which has the highest vapor pressure at
Q39: Which of the following is NOT an
Q45: According to your textbook, in ancient times,