The Kp for the reaction below is 1.49 × 108 bar-1 at 100.0 °C: CO(g) + Cl2(g) → COCl2(g)
In an equilibrium mixture of the three gases, PCO = 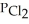 = 2.22 × 10-4 bar. The partial pressure of the product, phosgene (COCl2) , is ________ bar.
= 2.22 × 10-4 bar. The partial pressure of the product, phosgene (COCl2) , is ________ bar.
Definitions:
Compounded Monthly
A method of calculating interest in which the interest is added to the principal amount on a monthly basis and each subsequent interest calculation is made on the increased principal.
Compounded Quarterly
A method where interest is added to an investment or loan balance three months, leading to an increase in the total amount over time due to the effect of compound interest.
Promissory Note
A legal document in which one party promises to pay a specified sum of money to another party under agreed-upon terms.
Discounted
The reduction of the nominal price of goods, services, or financial instruments.
Q20: A 1.00 L buffer solution is 0.250
Q38: Consider the following reaction at equilibrium. What
Q43: Determine the molar solubility of BaF<sub>2</sub> in
Q67: If the pK<sub>a</sub> of HCOOH is 3.74
Q76: Ethanol (C<sub>2</sub>H<sub>5</sub>OH)melts at -114 °C. The enthalpy
Q91: Consider the following reaction: 2H<sub>2</sub>O(g)+ 2SO<sub>2</sub>(g)⇌ 2H<sub>2</sub>S(g)+
Q95: Given the following balanced equation, determine the
Q97: pH = pK<sub>a2</sub><br>A)equivalence point of a weak
Q104: Which one of the following has the
Q127: Determine the molar solubility of CuCl in