Determine the ammonia concentration of an aqueous solution that has a pH of 11.00. The equation for the dissociation of NH3 (Kb = 1.8 × 10-5) is below: 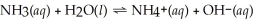
Definitions:
Shortcomings
Failures to meet a certain standard, often referring to weaknesses or flaws in something or someone.
Myocardial Infarction
A medical term for a heart attack, resulting from the interruption of blood supply to a part of the heart.
Behavioral Risk Factors
Characteristics or behaviors that increase the likelihood of developing a disease or injury.
Cardiovascular Disease
A class of diseases that involve the heart or blood vessels, leading to conditions like heart attacks or strokes.
Q5: What is the pH of a solution
Q9: Calculate the freezing point of a solution
Q20: Lewis base<br>A)proton donor<br>B)electron pair acceptor<br>C)proton acceptor<br>D)electron pair
Q44: The elementary reaction 2NO<sub>2</sub>(g)→ 2NO(g)+ O<sub>2</sub>(g)<br>Is second
Q49: Which of the following is the strongest
Q89: Determine the pH of a 0.023 mol
Q102: For the galvanic cell reaction, expressed below
Q123: Sketch the titration curve for a monoprotic
Q135: Which of the following is the correct
Q150: A 100.0 mL sample of 0.10 mol