A 0.25 M aqueous solution of potassium chloride,KCl,is tested for conductivity using the type of apparatus shown.What do you predict will happen? 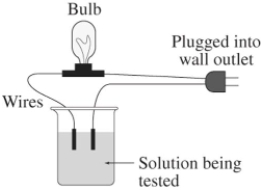
Definitions:
Variance
A measure of the dispersion, indicating how far individual numbers in a set are from the mean.
Increase
A rise or growth in quantity, size, number, value, or some other measure.
Binomial Distribution
A probability distribution that describes the number of successes in a fixed number of independent Bernoulli trials with the same probability of success.
Mean
The arithmetic average of a set of numbers, calculated by dividing the sum of these numbers by the count of the numbers in the set.
Q6: Nathaniel Wyeth developed plastic soft drink bottles.They
Q14: Which of the following ways of modifying
Q19: A chef tastes a soup and finds
Q20: Identify groups of structural isomers among the
Q22: Which statement explains why measurement by mass
Q33: The common OTC medication ibuprofen is sold
Q44: Which of the following describes a basic
Q45: What is meant by malnutrition?<br>A)More calories than
Q47: Isopentyl acetate (shown here) is used as
Q134: The Affordable Care Act requires all individuals