Use the data given below to construct a Born-Haber cycle to determine the lattice energy of CaO. DH°(kJ)
Ca(s) → Ca(g) 193
Ca(g) → Ca⁺(g) + e⁻ 590
Ca⁺(g) → Ca2⁺(g) + e⁻ 1010
2 O(g) → O2(g) -498
O(g) + e⁻ → O⁻(g) -141
O⁻(g) + e⁻ → O2⁻(g) 878
Ca(s) + 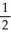 O2(g) → CaO(s) -635
O2(g) → CaO(s) -635
Definitions:
Scope of Employment
The range of actions and activities an employee is expected to perform as part of their job.
Plant Nursery
A specialized facility where plants are propagated, grown to a desired age, and then sold for planting elsewhere.
Liable
Legally responsible for one's actions or obligations, often in the context of paying damages or fulfilling a contractual agreement.
Durable Power of Attorney
A legal document granting someone the authority to act on another's behalf in legal and financial matters, which remains effective even if the grantor becomes incapacitated.
Q45: The hybrid orbital set used by the
Q53: Give the number of electrons in Na<sup>+1</sup>.<br>A)
Q57: Calculate the molar mass of Ca<sub>3</sub>(PO<sub>4</sub>)<sub>2</sub>.<br>A) 87.05
Q63: How many milliliters of a stock solution
Q68: Identify the number of electron groups around
Q70: Give the number of valence electrons for
Q80: Describe the reaction of the alkali metals
Q82: Identify a carboxylic acid.<br>A) CH<sub>3</sub>CH<sub>2</sub>OCH<sub>2</sub>CH<sub>3</sub><br>B) CH<sub>3</sub>CH<sub>2</sub>Cl<br>C) CH<sub>3</sub>CH<sub>2</sub>NH<sub>2</sub><br>D)
Q102: Which of the following elements is a
Q102: When waves of equal amplitude from two