Calcium oxide reacts with water in a combination reaction to produce calcium hydroxide: CaO(s) + 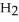 O(l) →
O(l) → 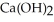 (s)
(s)
A 5.00-g sample of CaO is reacted with 4.83 g of 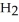 O.How many grams of water remain after the reaction is complete?
O.How many grams of water remain after the reaction is complete?
Definitions:
Customer Value
The perception of what a product or service is worth to a customer versus the possible alternatives, often influencing their loyalty and purchasing behavior.
Average Cycle Time
The average duration it takes to complete a process from start to finish, often used in manufacturing and project management.
Total Processing Time
The complete amount of time required to finish a task or process from start to end.
Value Of Goods
The monetary worth assigned to products, determined by factors such as market demand, production costs, and competitive pricing.
Q27: What element is undergoing oxidation (if any)in
Q37: What reagent could NOT be used to
Q39: A 35.6 g sample of ethanol (C<sub>2</sub>H<sub>5</sub>OH)is
Q79: Give the equation with the elements in
Q88: Define chemical energy.
Q96: How many milliliters of 0.260 M Na<sub>2</sub>S
Q128: Determine the electron geometry (eg)and molecular geometry
Q146: The following reaction is used to generate
Q152: Give the formula for calcium bisulfate.<br>A) CaHSO<sub>4</sub><br>B)
Q170: If the pressure in a gas container