A solution is 0.200 M in acetic acid and 0.100 M KCl.Calculate the concentration acetic acid and acetate in solution for a pH 4.500. 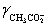 = 0.775,
= 0.775, 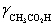 = 1,
= 1, 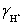 = 0.83 and Ka = 1.75 × 10−5.
= 0.83 and Ka = 1.75 × 10−5.
Definitions:
Leverage
The use of borrowed funds to enhance the potential return of an investment.
Transaction Costs
Expenses incurred when buying or selling securities, including broker fees, commissions, and other charges that affect the profitability of investments.
Forward Contracts
Non-standardized contracts between two parties to buy or sell an asset at a specified future time at a price agreed upon today.
Organized Exchange
A regulated marketplace for the trading of securities, commodities, derivatives, and other financial instruments.
Q9: A transmittance of 0.3847 is measured for
Q9: There are seven common strong acids.Which of
Q9: For the statements below,which is(are)TRUE for confidence
Q14: For Ag<sup>+</sup> when <font face="symbol"></font> = 0.01
Q16: Which of the responses below are sources
Q18: A sodium selective electrode increased in voltage
Q71: For markets to work well,there must be<br>A)
Q120: The basic principles of economics suggest that<br>A)
Q179: For most students,the largest single cost of
Q188: When the government implements programs such as